Chlorine in water is the weapon of choice for an array of applications ranging from cooling towers to water treatment for drinking standards. It has been used over a 100 years for water purification. It is fairly effective against Legionella bacteria if used in the correct way.
One factor to consider is water pH:
- In the swimming pool industry, they refer to controlling the pH as balancing.
- Cooling tower treatment providers regularly monitor pH due the nature of recirculating water, evaporation and atmospheric which influences concentrate the pH and alkalinity. Bromine, whilst not acceptable for drinking water, is often used as it is more compatible with these conditions.
- In drinking water, we don’t often consider the pH and its effect on chlorine based disinfectants. Australian water chemistry can vary immensely based on a variety of factors such as the source being a borehole, well or rainwater or the way the water is stored, many of which are unavoidable.
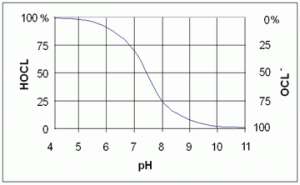
Chlorine, as a disinfectant, relies on the presence of two parts Hypochlorous Acid (HOCL) and Hypochlorite Ions (OCL-). Hypochlorous Acid is biocidal, so pH has a direct impact on their ratio. In the dissociation curve (see below) you can see the ratio change at the different pH. So in reality if the pH is out of the desired range your treatment could be rendered ineffective.
In buildings with little used areas like a vacant office floor or redundant pipework such as the long forgotten about office shower. The biocidal effects of chlorine may not reach these areas being it is a treatment dependant on the physical flow of water.
Chlorine is used by many drinking water utility companies but another compound, chloroamines is often employed. This is a product of combining ammonia with chlorine. The advantage of chloroamine over chlorine is it remains in solution for a longer period as chlorine is vulnerable to dissipating in solution. Chloroamines can sometimes be depleted and metabolised by nitrifying bacteria which can lower the alkalinity and pH of water, making it more acidic and possibly attack the metal parts of a plumbing network.
As well as water chemistry, another variation that can occur within water is the microbial composition. In peak summer the ambient water temperature is over 20℃ so you can expect bacteria numbers to increase rapidly. A common occurrence with older water distribution systems is the formation of biofilm on the internal surfaces of pipes. Biofilm is a slimy polysaccharide based substance created by bacteria which creates an ideal habitat for Legionella bacteria to thrive and offers protection from chlorine sometimes even when hyperchlorinated.
Looking at the limitation of chlorine as a disinfectant, it is always worth considering the water chemistry. There are many forms of treatment available on the market- including UV, silver-copper ionisation, chlorine dioxide and ozone but you must appreciate the operating requirements of each option. Every situation has a unique set of factors that must be taken into account when assessing and maintaining the water quality of a site. If you are having problems maintaining good water quality please contact compliance water services. ![]()



